What is the primary role of electrolytes in maintaining fluid balance?

Fluid balance is a cornerstone of overall health, vital for everything from nutrient transport to waste removal and maintaining blood pressure. At the heart of this intricate system are electrolytes – minerals that carry an electric charge when dissolved in body fluids. Their primary role in maintaining fluid balance is not just significant, it’s absolutely essential for life.
What Are Electrolytes?
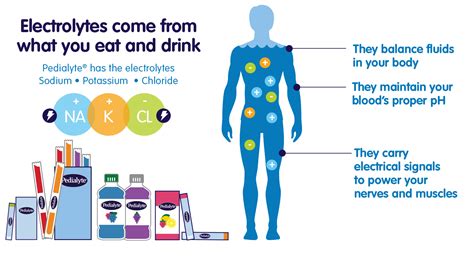
Electrolytes are substances that produce an electrically conductive solution when dissolved in water. In the human body, these are primarily mineral salts such as sodium (Na+), potassium (K+), chloride (Cl-), calcium (Ca2+), magnesium (Mg2+), phosphate (HPO42-), and bicarbonate (HCO3-). These charged particles are distributed throughout the body’s fluid compartments, including intracellular fluid (fluid inside cells) and extracellular fluid (fluid outside cells, such as plasma and interstitial fluid).
The Core Role: Osmosis and Water Movement
The fundamental mechanism through which electrolytes regulate fluid balance is osmosis. Osmosis is the movement of water across a semipermeable membrane from an area of lower solute concentration to an area of higher solute concentration. Electrolytes, being solutes, determine the osmotic pressure of a fluid compartment.
When the concentration of electrolytes changes in one compartment, water will move to equalize the solute concentration on both sides of the membrane. For instance, if the extracellular fluid has a higher concentration of electrolytes, water will shift out of cells into the extracellular space. Conversely, if the extracellular fluid has a lower concentration, water will move into the cells. This constant balancing act ensures that cells maintain their proper size and function, preventing them from swelling or shrinking excessively.
Key Electrolytes and Their Specific Functions
While all electrolytes contribute to osmotic balance, certain ones play more prominent roles in specific fluid compartments:
-
Sodium (Na+)
Sodium is the most abundant cation (positively charged ion) in the extracellular fluid. It is the primary determinant of extracellular fluid volume and osmotic pressure. Changes in sodium levels significantly impact blood volume and blood pressure. The body tightly regulates sodium concentration through various mechanisms, primarily involving the kidneys.
-
Potassium (K+)
Potassium is the primary cation within the intracellular fluid. While sodium dictates fluid outside cells, potassium is crucial for fluid balance inside cells. It’s also vital for nerve impulse transmission, muscle contraction (especially heart muscle), and maintaining normal blood pressure.
-
Chloride (Cl-)
Chloride is the most abundant anion (negatively charged ion) in the extracellular fluid. It often moves with sodium to maintain electrical neutrality and plays a role in regulating fluid shifts between compartments.
-
Calcium (Ca2+) and Magnesium (Mg2+)
These electrolytes are essential for bone health, muscle function, nerve transmission, and various enzymatic reactions, indirectly contributing to overall fluid and cellular health.

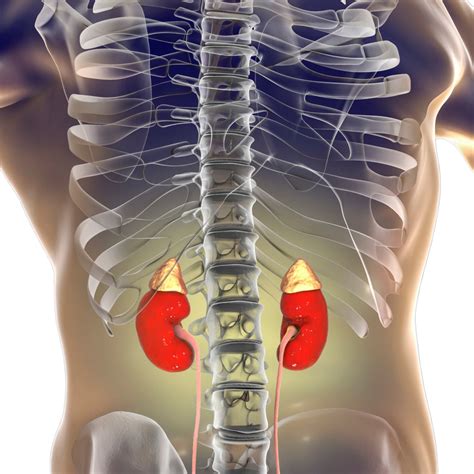
Maintaining Equilibrium: The Kidney’s Role
The kidneys are the primary organs responsible for regulating electrolyte concentrations and overall fluid balance. They selectively filter, reabsorb, and excrete water and electrolytes to maintain homeostasis. Hormones like antidiuretic hormone (ADH) and aldosterone also play critical roles in regulating water reabsorption and sodium-potassium balance, respectively, thereby directly influencing fluid volume and electrolyte concentrations.
Consequences of Electrolyte Imbalance
An imbalance in electrolytes, whether too high (hyper-) or too low (hypo-), can have serious health consequences. Conditions like dehydration (loss of fluids and electrolytes) or overhydration (excess water intake diluting electrolytes) can lead to symptoms ranging from muscle cramps and fatigue to more severe issues like cardiac arrhythmias, seizures, and even coma. Maintaining proper electrolyte levels through adequate hydration and a balanced diet is therefore paramount.

Conclusion
In essence, electrolytes are the conductors of the body’s fluid orchestra. Their ability to generate osmotic pressure dictates where water goes, ensuring that every cell and organ receives the necessary hydration to function optimally. From controlling nerve impulses and muscle contractions to maintaining blood pressure and cellular integrity, the primary role of electrolytes in fluid balance is foundational to human physiology and well-being. Understanding their importance underscores the necessity of proper hydration and a diet rich in essential minerals.








